By Lilly Hancock
Language both connects us and separates us. The words we use, their meaning, and how we interpret or misinterpret them greatly impacts our understanding of the world. This truth became outwardly apparent when trying to write about greenhouse gases, natural climate solutions, net-neutrality, and the various carbon marketplaces. To write comprehensively about these systems, I found myself leaning into language, content, and natural processes that few people understand or possibly haven’t thought about since 10th grade biology class. It occurred to me that maybe, if we all had a better understanding of biology and Earth systems, we’d see climate change (and our impact on it) more rationally.
So, I decided to start with carbon—both because I spent much of my career researching how plants photosynthesize and because the word carbon and its properties hold a lot of power. Carbon is the core structural element and “food” for plants, a product of animal respiration, the predominant greenhouse gas, and fundamental to buzz words such as “net-zero”, “carbon off-sets”, and “Payments for Ecosystem Services”.
I believe a shared understanding of carbon has the power to demystify: if society at large understood the carbon cycle, perhaps the idea of fossil fuel combustion resulting in rapid atmospheric change could be more of a logical deduction and less of a political football. The text below is by no means comprehensive, rather my goal here is to define a common language so that we can eventually discuss natural climate solutions, the pros and cons of carbon credits, and the possibility of turning our soils into a carbon sink, along with other possible approaches to dealing with the current climate crisis.
Carbon Dioxide and the Carbon Cycle
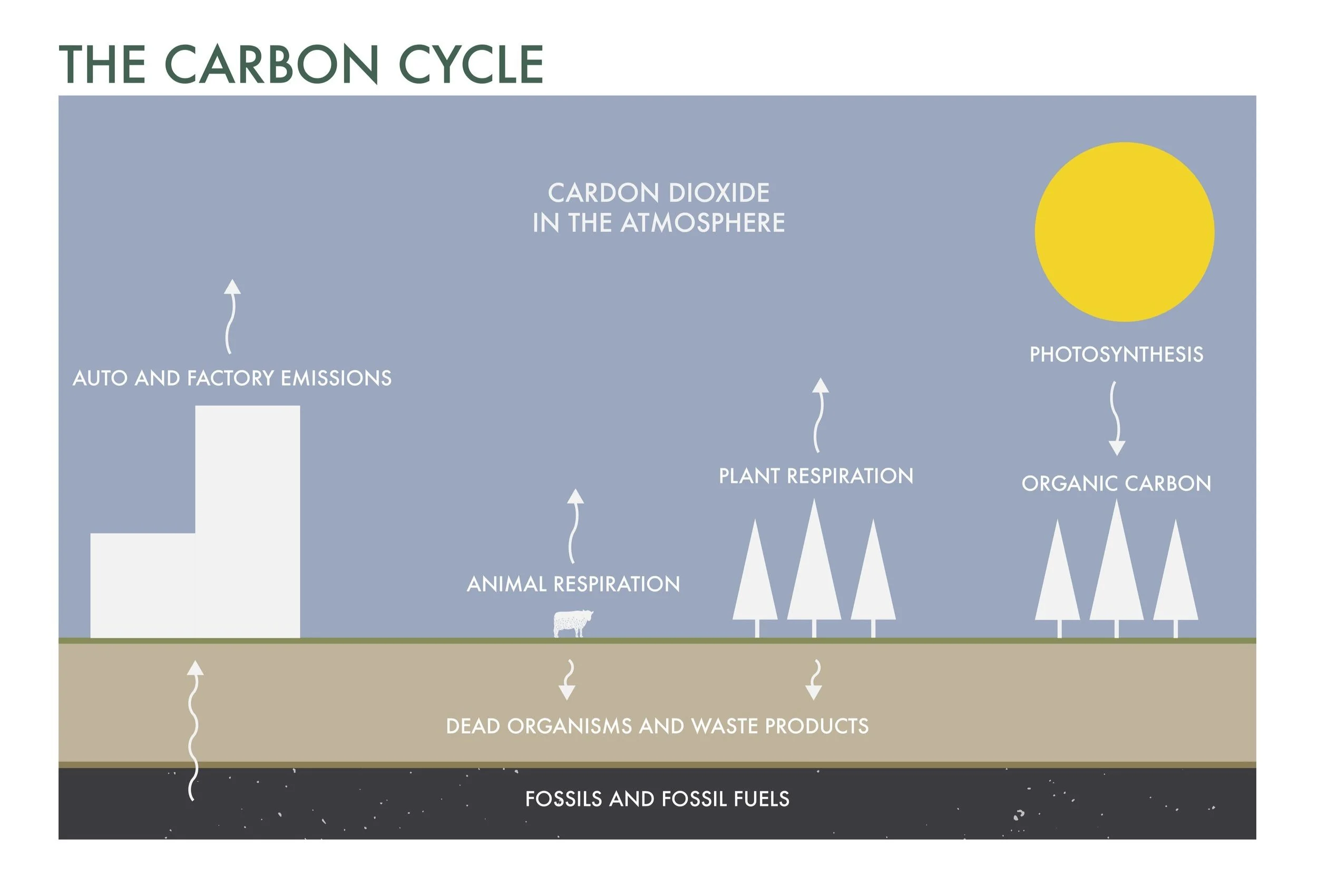
Let’s start at the beginning. Carbon is the core structural element of the earth’s lifeforms. Its molecular structure allows it to bind easily to other elements, creating large complex molecules that give plants and animals solid bodies as well as small, light molecules that make up our atmosphere, notably carbon dioxide (CO2). In its gaseous form, something remarkable happens— plants, algae, and cyanobacteria grab this CO2 (along with water and sunlight) and turn it into energy-rich organic molecules (e.g., sugars), essentially creating their own food from the air. We call this amazing process photosynthesis. Plants use these carbon-based sugars to store energy so they can grow and reproduce. In addition, some of this carbon is secreted by the roots, feeding soil organisms, and promoting the formation of soil organic matter which is the basis of soil fertility.
Where does all this CO2 in the atmosphere generally come from? Geologists estimate that large quantities of CO2 were seeded into our early atmosphere by volcanos (this process still happens, though there are much fewer volcanos today). In the earth’s modern era, CO2 is mostly released into the atmosphere through processes of life’s carbon cycle: primarily decomposition, respiration, and combustion.
A good way to think about the carbon cycle is by considering sources and sinks. Sources release carbon into the atmosphere: decomposition of organic matter by fungi and bacteria, respiration by animals (we breathe out CO2), and combustion of organic matter (fire). Sinks, on the other hand, take carbon out of the atmosphere and store it for varying amounts of time. Geologic processes are the biggest and slowest; the creation of coal and shale by deposition and burial, as well as limestone in tropical oceans, stores carbon for millions of years, but these are extremely slow processes. The ocean is a massive sink that cycles carbon on the order of 10s to 1000s of years, with plankton, algae, and other heterotrophic organisms being responsible for CO2 capture. Plants are a large sink that cycle carbon on the scale of years, with trees being the most effective because of their long lifespans. Another natural sink is soil.
Soil carbon sequestration is the process by which CO2 is removed from the atmosphere by plants and then stored in the soils as soil organic carbon (SOC). Unfortunately, agricultural activities (tillage, erosion, etc.) over the last 150 years have led to the depletion of SOC, turning our soils from a sink into a source. This can (and needs to) change not only from a climate perspective but also from a food security perspective.
The concentration of CO2 in the atmosphere at any given moment is largely dependent on the balance of these vectors: sources releasing carbon into the atmosphere and sinks pulling it down to store it for a while in solid matter. This balance has always been shifting, as we know from the dynamic geologic record of warm, globe-spanning jungles (the real Jurassic park) and ice ages. What is notably different today is how fast CO2 in the atmosphere is increasing and how fossil fuels are to blame.
Fossil fuels: short-circuiting the carbon cycle
The term fossil fuels might conjure up the Sinclair gas station logo and our favorite dinosaurs, but these carbon-rich deposits are really the fossilized remains of something more like mud: thick mats of dead vegetable matter that collected in bogs and swamps during the geologic periods known collectively as the “Carboniferous” (and a few younger periods). Not all muddy bogs and river bottoms get buried, but some huge ones did: these massive carbon sinks got covered by more sediment and pushed down deep into the earth, deep enough to be baked by the earth’s heat into shale, mudstone, and coal. The important thing to consider is that these layers of rock, today’s coal seams and shale beds, are made of carbon from 200-400 million years ago that’s been locked away all this time.
No sink lasts forever and there is a slow process by which coal and shale release their carbon into the atmosphere. But humans, in a stroke of ingenuity that unleashed the industrial revolution, figured out how to release the energy stored in that ancient carbon all at once: combustion. First, we learned to burn coal in furnaces, then gasoline in engines, then natural gas in homes and generators. This is why the concentration of CO2 has been increasing so rapidly in the last 200 years: besides the natural cycle of sources and sinks, we’ve tapped into a mountain of ancient stored energy, effectively short-circuiting the carbon cycle and turning a long-term sink into a source. In essence, we’ve massively increased the sources, and the natural sinks just can’t keep up.
Environmentalists, farmers, engineers, scientists, and climate activists are working tirelessly to increase our natural carbon sinks through both the sequestration of carbon and reduction of emissions. Such natural climate solutions encompass reforestation, avoided forest conversion, fire management, conservation agriculture, agroforestry, grazing and animal management, and coastal restoration, to name a few. All of these solutions tap into the carbon cycle and aim to optimize how carbon is stored or held in various sinks. It is hypothesized that even if we are able to maximize these natural climate solutions, we will only realize a portion of the climate goals set forth in the Paris Climate Agreement. And that’s not surprising when you think about it, the amount of CO2 in our atmosphere has nearly doubled in the last 200 years.
One component that hasn’t been addressed yet (but is critical to an overall understanding of climate change) is how exactly an increase in CO2 and other greenhouse gasses (methane, nitrous oxide, and fluorinated gases) change the earth’s climate. Most sunlight (~70%) is absorbed by the earth, and as the rocks, land, air, and oceans warm they radiate energy as heat (thermal infrared radiation). This radiant heat travels into the atmosphere where is absorbed by water vapor and greenhouse gasses. These water and gas molecules, in essence, function as tiny heaters, trapping heat and radiating it in all directions, one of which is back towards earth. So, an increase in greenhouse gases means more trapped energy, causing an increase in global temperatures. It may seem small, but an average global shift of 1°C can be catastrophic and raise sea levels, shift ocean currents, alter precipitation patterns, decrease habitat, result in biodiversity loss, and jeopardize human civilization.
The margin for error, as it relates to climate change mitigation, is slim. Prior to 2014 and the Paris Climate Agreement, scientists predicted a global temperature increase of 4°C (7.2°F) by 2100. Due to rapid growth in clean energy (solar, hydro, wind), we have started to curve emissions, putting us on pace for a 3°C rise by 2100. But we must move faster and there are ways to do just that.
For better or worse, I’m going to brush over the most obvious and the most impactful, the burning of fossil fuels, because it is both obvious and complex. Instead, I’ll seed a follow-up conversation (in the form of a blog), that touches more specifically on natural climate solutions, particularly as they relate to agriculture.
How we grow our food has a profound impact on our climate. Planting crops and rearing animals releases greenhouse gases into the air, while converting land from forest to agriculture turns carbon sinks into sources. With all that said, there are ways to farm and manage land that adds value to the system as a whole: farm management practices that build topsoil, add fertility, sequester carbon, create resiliency, reduce emissions, and produce nutrient-rich food. This all can be realized, and the data and marketplace required to support the transition away from conventional agriculture (as carbon source) to regenerative agriculture (a carbon sink) is building considerable steam.